(BIVN) – There were 156 newly reported cases of COVID-19 in the State of Hawaiʻi on Thursday, up from the 139 reported on Wednesday. There were 17 new cases identified on Hawaiʻi island, down from the 32 identified the day before. Four (4) new deaths with COVID-19 were reported statewide.
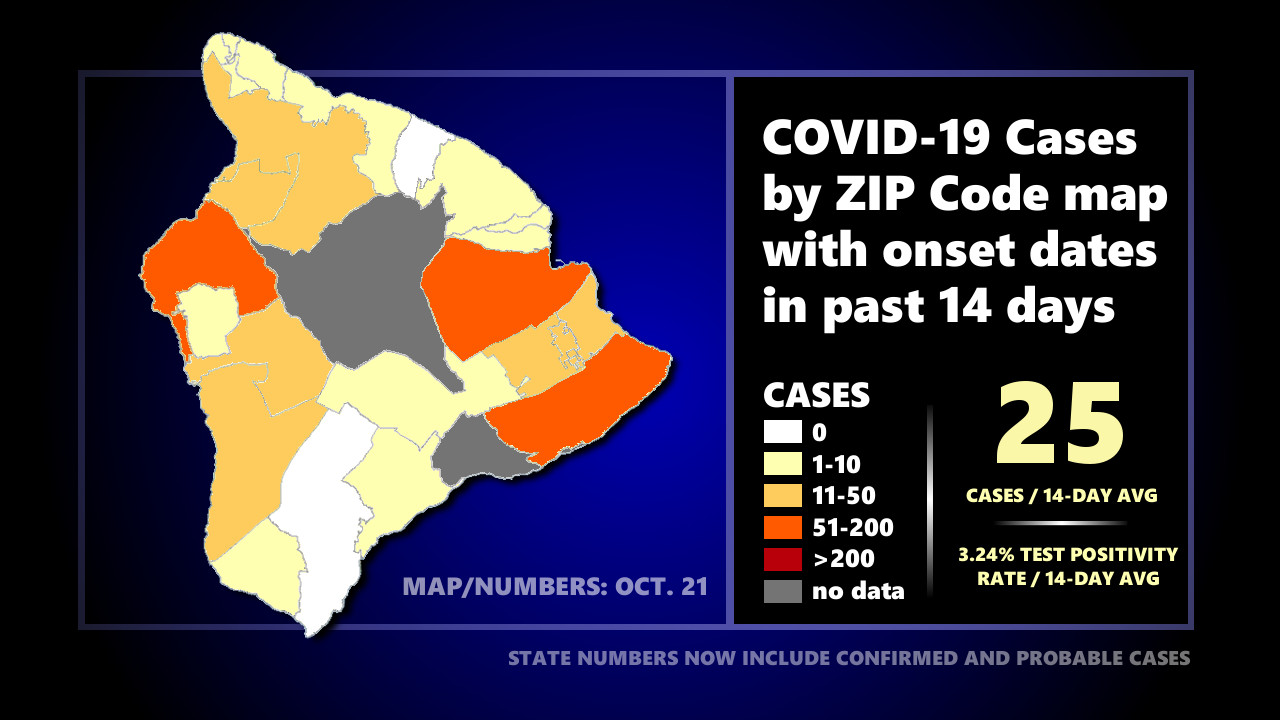
Health officials are currently monitoring 405 active cases on Hawaiʻi island. The test positivity rate in Hawaiʻi County over the last 14 days is 3.2%. There has been a 14-day average of 25 new cases per day on the Big Island.
On the Health Department’s zip code area map showing reported COVID-19 cases with onset dates in the past 14 days, there are ten (10) zip code areas on the Big Island showing over 10 cases. Three (3) of those zip code areas are showing over 50 cases. Zip code areas not listed below have recorded less than 10 cases in 14 days.
- 96720 (Hilo) – 84 cases
- 96740 (Kona) – 95 cases
- 96743 (Kohala) – 11 cases
- 96738 (S. Kohala) – 14 cases
- 96749 (Puna) – 32 cases
- 96760 (Puna) – 16 cases
- 96771 (Puna mauka) – 12 cases
- 96778 (Puna makai) – 62 cases
- 96750 (Kona) – 15 cases
- 96704* (South Kona) – 13 cases
* The 96704 zip code area includes the zip code area of 96726.
To date, the State of Hawaiʻi says 2,130,298 cumulative doses of COVID-19 vaccine have been administered. Health officials say 70.6% percent of the State population has been fully vaccinated. 79.1% of the population has initiated vaccination. On Hawaiʻi island, 66% has completed vaccination.
Moderna, Johnson & Johnson Vaccines Authorized
The Hawai‘i Department of Health said Thursday it will adopt the U.S. Center for Disease Control and Prevention’s guidelines on COVID-19 booster doses for Moderna and Johnson & Johnson vaccines.
“Boosters doses are common for many vaccines and will provide additional protection to Hawai‘i residents at higher risk for severe illness or occupational exposure,” said Director of Health Dr. Elizabeth Char, FACEP. “Boosters are expected to be widely available across the state, and CDC’s mix-and-match policy will allow for additional flexibility. DOH’s first priority will remain encouraging unvaccinated Hawai‘i residents to complete their initial vaccine series.”
From the Hawai’i DOH:
Moderna vaccine recipients: A single booster dose is recommended for certain populations at least six months after the second dose:
1.) Individuals 65 years and older
2.) Individuals age 18+ who live in long-term care settings
3.) Individuals age 18+ who have underlying medical conditions
4.) Individuals age 18+ who work or live in high-risk settingsModerna booster doses are half of an initial dose.
Johnson & Johnson vaccine recipients: A single booster dose is recommended for all Johnson & Johnson recipients at least two months after the first dose. Johnson & Johnson recipients can also elect to receive a single booster dose of the Pfizer or Moderna vaccines.
Mixing and matching of all U.S.-approved COVID-19 vaccines is allowed. Interchangeability of shots will provide additional flexibility. All vaccines are effective in reducing the risk of severe disease, hospitalization and death, even against the Delta variant.
First and second doses will continue to be prioritized over any booster doses. The best way to protect Hawai‘i families and communities is to ensure that unvaccinated Hawai‘i residents complete their initial vaccine series.
Individuals unsure if they qualify for a booster should check with their healthcare provider.
Individuals are still considered fully vaccinated 14 days after their primary vaccine series. Booster doses provide additional protection, but the primary series continues to protect vaccinated individuals against severe illness, hospitalization and death.
Hawaiʻi Prepares For Vaccines For Keiki 5-11
The Hawai‘i health department also said Thursday that it is planning for the “likely administration” of COVID-19 vaccinations to children 5-11, as it closely monitors federal review of the Pfizer vaccine for this age group. From the DOH:
The U.S. Food and Drug Administration (FDA) must authorize use of the vaccine and the U.S. Centers for Disease Control and Prevention (CDC) must issue recommendations for clinical use of the vaccine before administration can begin. However, DOH is preparing to make Pfizer’s vaccine for children available statewide in the event its use is authorized.
“DOH has been working with public- and private-sector partners to prepare for the likely authorization of COVID-19 vaccines for children 5-11 to ensure equitable distribution across the state,” said Health Director Dr. Elizabeth Char, FACEP. “While Pfizer reports its vaccine provides robust protection for children 5-11, we await a review of the scientific evidence by federal regulators. The lives of our keiki are precious, and we are encouraged we may soon be able to protect them from COVID-19 through vaccination.”
DOH estimates there are 119,473 children 5-11 living in Hawaiʻi, which is roughly 8.4% of the state’s population. An FDA advisory committee is scheduled to meet Oct. 26 to discuss authorization of the vaccine and a CDC advisory committee is scheduled to meet Nov. 2-3 to discuss clinical recommendations.
DOH is working with providers to offer vaccinations at a wide range of locations including schools, pop-up clinics, community health centers, hospitals, pharmacies and a limited number of pediatricians’ offices. Pediatricians and other medical providers can register here to administer COVID-19 vaccines. DOH also plans a comprehensive communication and outreach campaign to parents and guardians of children 5-11.
The federal government is expected to distribute the first waves of vaccine directly to states and DOH has pre-ordered the full allotment allocated to Hawaiʻi – 41,700 doses. This first allocation will cover 35% of Hawaii’s 5-11-year-old population. Doses will then be pre-positioned on each island based on 5-11-year-old population estimates. The Pfizer dose being considered for children 5-11-year-old is one-third of the adult dose and is expected to be administered with a smaller needle.
Upon authorization, a list of sites offering vaccinations to this group will be posted at HawaiiCOVID19.com/vaccine. Parents and guardians of 5-11-year-olds will need to fill out a written or electronic consent form before a vaccine can be administered.

by Big Island Video News8:32 pm
on at
STORY SUMMARY
HAWAI'I ISLAND - The State has authorized booster doses for Moderna and Johnson & Johnson vaccines, and is preparing for likely authorization for keiki 5 to 11 years of age.