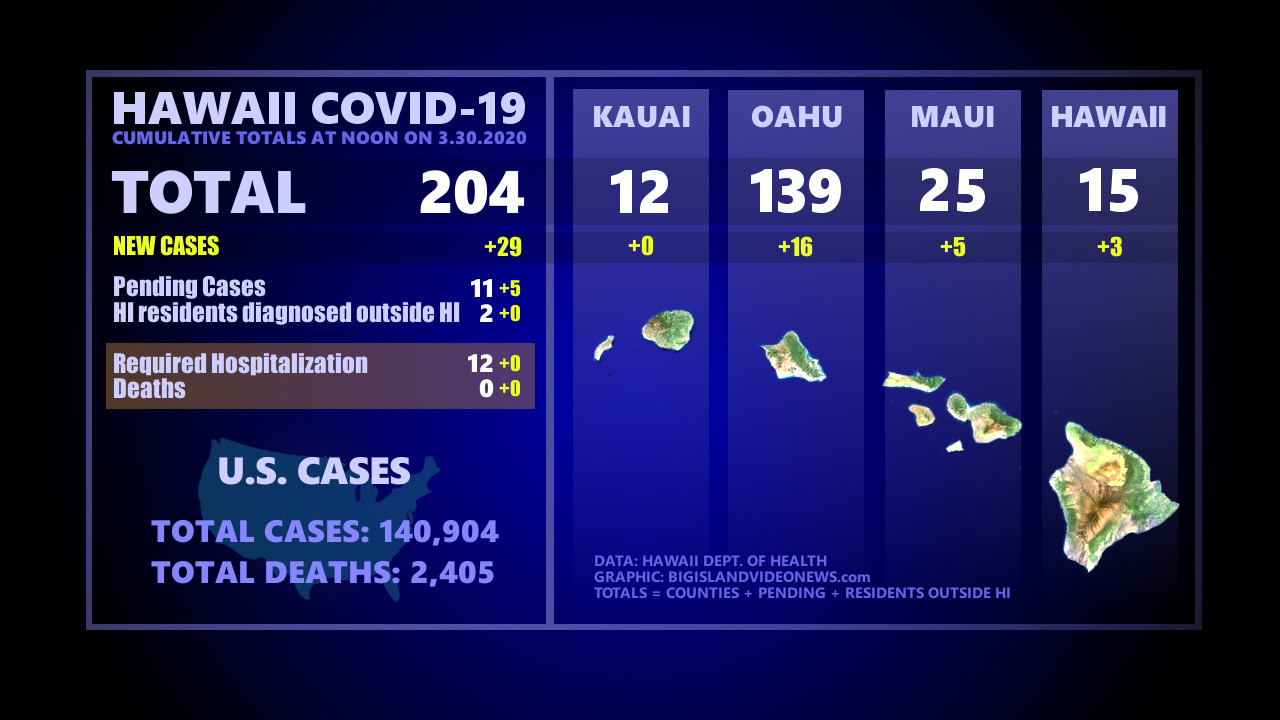
(BIVN) – Today, the Hawaiʻi Department of Health reported 204 positive cases of COVID-19 statewide. The cumulative total, which includes both presumptive and confirmed cases, has increased by 29 from yesterday.
There have been 15 cumulative cases identified on the Big Island, up three from yesterday. There continues to be a small discrepancy between the number reported by the state and the number reported by the county. At 9 a.m., Mayor Harry Kim said in a civil defense message that “the total number of people tested positive for Hawaii Island is 16 with 5 recovered. The remaining 11 are quarantine at home.”
There are 139 cases on Oʻahu, up 16. There are 12 on Kauai, and 25 on Maui (up 5).
Added to the total number are 11 pending cases and 2 Hawaiʻi residents diagnosed outside of Hawaiʻi.
12 have required hospitalization, and there have been no reported deaths from the virus in Hawaiʻi. Health officials say six more cases have been released from isolation, bringing the total to 55.
“Isolation should be maintained until at least 3 days (72 hours) after resolution of fever and myalgia without the use of antipyretics OR at least 7 days have passed since symptom onset, whichever is longer,” the state says.
SCHATZ: $4 Billion Coming To Hawaiʻi
U.S. Senator Brian Schatz (D-Hawai‘i) announced today that Hawai‘i is set to receive at least $4 billion in federal coronavirus relief funding.
“Billions of dollars in federal money is on the way,” said Senator Schatz, a member of the Senate Appropriations Committee. “This new funding will support state and local response efforts and help Hawai‘i families and businesses struggling to get by.”
Key funding for Hawai‘i includes:
- $1.25 billion to help fund state and county government response efforts;
- $1.14 billion in estimated unemployment assistance;
- $1.24 billion in estimated direct cash payments to Hawai‘i residents;
- $130 million in estimated funding for the Supplemental Nutrition Assistance Program (SNAP);
- $53 million to support local schools and colleges during the pandemic;
- $11 million for Hawai‘i’s community health centers;
- $8 million in Community Development Block Grants;
Sen. Schatz says millions more in federal money for Hawai‘i “will go to additional health care, education, public transportation, and housing programs.”
U.S. Food and Drug Administration Updates
(FDA media release with emphasis added by BIVN)
On March 28, 2020, the FDA issued an Emergency Use Authorization (EUA) to allow hydroxychloroquine sulfate and chloroquine phosphate products donated to the Strategic National Stockpile (SNS) to be distributed and used for certain hospitalized patients with COVID-19. These drugs will be distributed from the SNS to states for doctors to prescribe to adolescent and adult patients hospitalized with COVID-19, as appropriate, when a clinical trial is not available or feasible. The EUA requires that fact sheets that provide important information about using chloroquine phosphate and hydroxychloroquine sulfate in treating COVID-19 be made available to health care providers and patients, including the known risks and drug interactions. The SNS, managed by ASPR, will work with the Federal Emergency Management Agency (FEMA) to ship donated doses to states.
On March 29, 2020, the FDA issued an immediately in effect guidance that outlines an enforcement policy to help expand the availability and capability of sterilizers, disinfectant devices and air purifiers. The devices include those intended to make devices sterile, kill pathogens or other microorganisms and kill pathogens or microorganisms in the air. This policy reflects FDA’s commitment to ease burdens on health care providers and facilities as they face COVID-19.
The FDA amended the Emergency Use Authorization (EUA) for the Battelle Decontamination System for use in decontaminating compatible N95 respirators for reuse by health care personnel during the COVID-19 pandemic. This EUA is an important step forward in helping to reduce shortages in critical N95 respirators, by allowing for these important devices, when decontaminated, to be reused by medical professionals on the front lines of the COVID-19 pandemic.
On March 30, 2020, the FDA issued an immediately in effect guidance to help expand the availability of surgical apparel for health care professionals, including gowns (togas), hoods, and surgeon’s and patient examination gloves during this public health emergency.
FDA and FTC issued warning letters to two companies for selling unapproved products claiming to mitigate, prevent, treat, diagnose or cure COVID-19. One of the companies, Corona-cure.com, was warned for selling the product Coronavirus Infection Prevention Nasal Spray with misleading claims on its website that its product is safe and/or effective for the treatment or prevention of COVID-19. The agencies also warned Carahealth for selling its herbal products, including “Carahealth Immune,” with misleading claims of prevention and/or treatment of COVID-19. We are particularly concerned that unapproved drugs that claim to cure, treat, or prevent serious conditions may cause consumers to delay or stop appropriate medical treatment, leading to serious or life-threatening harm. There is currently no approved treatment or preventative measure for COVID-19. FDA and FTC are closely monitoring social media, the online marketplace, and incoming reports for fraudulent COVID-19 products on the market.
The FDA issued an updated guidance, “Conduct of Clinical Trials of Medical Products during COVID-19 Pandemic,” with an appendix adding questions and answers on this subject. We plan to update this appendix as new questions arise. This guidance is intended for industry, investigators and institutional review boards and was issued because we recognize that the COVID-19 pandemic may impact the conduct of clinical trials of medical products, including drugs, devices and biological products.
Diagnostics update to date: During the COVID-19 pandemic, the FDA has worked with more than 230 test developers who have said they will be submitting emergency use authorizations (EUA) requests to FDA for tests that detect the virus. To date, 20 emergency use authorizations have been issued for diagnostic tests, including Abbott Diagnostics Scarborough, Inc., ID NOW COVID-19, a rapid (13 minutes or less) test. Additionally, the FDA has been notified that more than 110 laboratories have begun testing under the policies set forth in our COVID-19 Policy for Diagnostic Tests for Coronavirus Disease-2019 during the Public Health Emergency Guidance. The FDA also continues to keep its COVID-19 Diagnostics FAQ up to date.
We will be updating this page with more information.

by Big Island Video News12:11 pm
on at
STORY SUMMARY
HAWAIʻI ISLAND - The state says there are 15 cumulative cases of COVID-19 on the Big Island, although the mayor says that number is 16.